Here is a quick rundown of what the government has said about the shots taken out of distribution after problems were found with vials.
Hong Kong residents who had already received their first shot of the BioNTech vaccine for the coronavirus reacted with shock and alarm on Wednesday morning when the government suspended the use of the jab after reports of packaging defects, but many felt they were still in the dark on what to expect next.
Of the 585,000 doses in the first batch dispensed, or batch 210102, about a quarter, or 150,000, had been used so far.
In Hong Kong, Fosun Pharma is in charge of delivering the jab jointly developed by Germany’s BioNTech and US-based Pfizer. The vaccines are made in Germany.
More than 50 defects that included cracks, leakages and stains outside the glass containers were reported, but all potentially spoiled vials had been disposed, health authorities said.
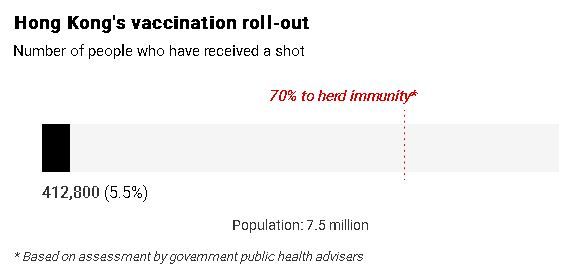
The abrupt halt has dealt another blow to public confidence in the city’s immunisation scheme - which has recorded only a vaccination rate of about 5 per cent - after several deaths among recipients, all with chronic diseases, were reported. No link between the deaths and the vaccines has been established.
The health minister stressed the halt was a precautionary measure given there was no evidence so far to suggest the shots posed a health risk. Here are some questions we have received from readers:
How serious were the packaging defects?
Director of Health Dr Constance Chan Hon-yee revealed there were eight incidents of cracked BioNTech vials and 22 air pressure issues resulting in leaks.
Another 16 reports of vial seals being loose or out of position were made, as well as 11 that were linked to the identification of stains or marks on the exterior of the glass containers.
She said all those vials in question had been disposed of after problems were identified and vaccines from those vials were not administered to the public.
Recipients can check their vaccination records to determine whether they had received a dose from the batch in question.
Are there any health risks for those who have already taken the first dose?
The manufacturers said they could not see any problems related to safety concerning the vaccines, but they would need to conduct a thorough investigation, according to Chan.
“For the sake of caution, they requested Hong Kong to suspend the use of this batch,” she said.
William Chui Chun-ming, president of the Society of Hospital Pharmacists of Hong Kong, said pharmacists were at community vaccination centres as gatekeepers.
“If you ask me whether the quality of the drugs had been affected, the chance is very low,” he said.
Chui said if cracks or leakage were found, medical staff would not use them.
For vial seals that were out of position or loose, as long as the rubber stopper remained tight and intact, the quality would not usually be compromised, he said. But this issue would need further clarification from the manufacturers.
Officers would also clean the stains on the exterior of the glass vials with alcohol, he added.
What next for those who have taken the first shot?
Do not take any action until you are further notified by the health authorities. Chan said that for now anyone who had received a first jab did not have to repeat the dose. Even though most people’s appointments for the second jab would be 21 days later - which had now been suspended - the follow-up shot of the BioNTech vaccine could be administered 19 to 42 days after the first injection, she added.
She also advised them against switching to another brand of vaccines for their second dose or retaking a first dose of another vaccine.
Hong Kong’s supply of batch 210104 was not yet in use and all of its 758,000 doses were in storage.
Chan said the users of the vaccine most urgently affected would be those needing to receive their second dose on Saturday. They would have been the first to get the BioNTech shots nearly three weeks ago.
“If the current stock in Hong Kong cannot be used, we would request the pharmaceutical firm to deliver another batch as soon as possible,” she said.
What about those who have registered for BioNTech for the coming days and weeks?
The programme is suspended for now. Secretary for Civil Service Patrick Nip Tak-kuen, who oversees the vaccination campaign, said the online booking system for the German-made drugs had been suspended and the arrangements for residents to change their appointment would be announced later.
How did recipients in the city react to the suspension?
Most had more questions than authorities seemed able to answer for now. Jez Yong, 32, who works in a consultancy firm and just received the first BioNTech dose on Tuesday morning, spoke for many when he asked about the potential problems that could arise from the defective packaging.
“Like lower effectiveness? Would the second jab mean that wouldn’t be an issue?” he said.
Musician Richard Fong To, 45, who received his dose last Tuesday, said it was right to suspend the vaccine given the problems. But he criticised the government for what he described as double standards when dealing with the vaccines.
“There were seven people who died after receiving the Sinovac jab, but it doesn’t suspend the vaccination. When there are packaging defects, you then ask it to halt,” he said. The authorities have not established any link between the deaths and the Sinovac vaccine as all had pre-existing chronic conditions.
Will Hong Kong have enough supply of vaccines?
The city has struck deals to purchase 22.5 million doses of vaccines, with 7.5 million shots each coming from three suppliers: Sinovac Biotech; British-Swedish firm AstraZeneca; and Fosun Pharma, which is delivering the jab jointly developed by BioNTech and Pfizer.
More than 2.3 million doses of Sinovac and BioNTech vaccines have been shipped to the city.
Earlier, the government estimated AstraZeneca jabs would arrive in the second half of this year, but no concrete timetable was given, while local officials said they would monitor possible side effects of the vaccine after reports of blood clots in some recipients in Europe.
Are there initial theories on the cause of the damaged vials and how long will the investigations take?
The BioNTech shots must be kept at minus 70 degrees Celsius and thawed and diluted before injection. Each inoculation centre is equipped with two pharmaceutical fridges to store the vials at temperatures between 2 and 8 degrees for no more than five days. The prepared solutions must be used within six hours and cannot be stored above 30 degrees.
The authorities could not say whether the damaged containers had anything to do with the storage and transportation of the vaccines.
Fosun Pharma, which is distributing the BioNTech jabs in China, pledged to conduct an immediate investigation, covering areas including the manufacturing process and logistical operations. But there was no timeline on when Fosun and BioNTech would complete their investigation into the defects.
Professor Ivan Hung Fan-ngai, co-convenor of an expert committee monitoring the side effects of vaccines, said that based on current reports the problems involved just minor defects which were unlikely to affect the vaccination programme in the long run.
“Once we have sorted this incident out and also confirmed that’s just a minor defect, I am sure that the vaccination programme will resume as soon as possible,” he said.
As of 8pm on Wednesday, official figures showed a total of 412,800 people, or about 5.5 per cent of the city’s population, had been vaccinated. Of those, 151,300 had received the first dose of the BioNTech vaccine, compared with 261,500 for the Sinovac one.















